Knowledge base » Release Notes - EpiSoft/CareZone » 2021/01/27 Release Notes - Clinical
2021/01/27 Release Notes - Clinical
Active Ingredient Prescribing – Mandatory legislative changes under the PBS
This is a legislative requirement as at 1 Feb involving major changes to the medication record and the protocol module. The purpose of the legislation is to encourage generic prescribing (rather than brand medications) where it is considered safe to do so.
The ‘where it is considered safe’ has resulted in a number of drugs falling into two exemption categories:
The List of Excluded Medicinal Items (LEMI) means the drug is exempt from including generic name on the script at all. This includes drugs with four or more active ingredients, a number of vaccines, allergens and other assorted formulations. If prescribed by brand, only brand will present on the printable script. If prescribed by generic, there will be a prompt to consider prescribing by brand instead.
The second exemption category – List of Medicines for Brand Consideration (LMBC) is where brand inclusion is recommended for clinical reasons. If prescribed by brand, both brand and generic will present on the printable script for an LMBC drug. If prescribed by generic, there will be a prompt to consider prescribing by brand.
The majority of drugs fall into neither exemption category, which means as a general rule, there will be less alerts on the whole if you record and prescribe medications by generic. If you prescribe by generic, there will be overall fewer prompts and changes to your use of either the main medication or protocol module. If you prescribe by brand, you will be prompted to confirm brand name is clinically necessary. If you decide that it is, a script for brand medication will also include generic name which will appear in front of the brand name on the printed script. This is part of the mandatory legislative requirement (that the generic is on the printed script for all drugs other than LEMIs – see above for definition) so please check your printed scripts as the vast majority of new scripts created from this date onwards will now print both generic and brand.
MIMS is providing the flags that cover the above two exemptions so as you select a drug from MIMS, the check will occur to determine whether the drug has one of these exemption flags or not. There are also of course thousands of medications already recorded and/or published in a protocol definition that are part of current medical records that could also be impacted so we have flagged these in the respective modules for you to modify or decide to proceed with current format before you get to generating the script.
How has this legislative requirement been implemented in EpiSoft?
Medication form changes
On the medication (non protocol) form, historically recorded medications will display an alert if they have been recorded in a method not compatible with the Active Ingredient Prescribing regulations. This is to assist you in making your decision regarding brand or generic before you generate a new script for those drugs. See below.

If you decide to keep the medication as is, click the alert icon and then OK to keep your generic or brand decision as is, otherwise selecting cancel will open the medication in Edit mode and you can reselect the drug by generic name (or vice versa).

If you have decided to retain brand or generic as per the patient’s current list, overwriting the AIP recommendation, a green tick will show this choice has been made for this patient and that drug and the tooltip will show who ticked this and when.

If you go to write a script without actioning these alerts, the script module will prevent you from proceeding until a decision has been made (or the drug re-selected).

When you go to record a new medication, you will get a prompt as shown below. While we recognise that this also impacts sites that are merely recording current medications (and not writing prescriptions), we have included this prompt at the time of medication recording to reduce prompts and alerts at the time of script writing.

Protocol Module changes:
When you go to record a new medication, you will get a prompt as shown below. While we recognise that this also impacts sites that are merely recording current medications (and not writing prescriptions), we have included this prompt at the time of medication recording to reduce prompts and alerts at the time of script writing.
There are a number of drugs normally prescribed by generic which are actually flagged as LMBC (List of Medicines for Brand Consideration) so this will comprise the bulk of the AIP alerts (examples rituximab, trastuzumab (not all formulations), zoledronic acid.
For this reason and because the LMBC inclusion is strongly recommended (not mandated), we have ticked all LMBC drugs by default so when you save New Protocol or “Apply Medication Selection” on Edit protocol, this default tick will be saved.
To reiterate, if the patient is already on a protocol with AIP alert listing as below and the drug has been auto-ticked, you will still need to hit Apply Medication Selection to commit that default to the patients current and future protocol cycles.
The other items which are LEMI or branded medications where generic is recommended will need to be actively ticked (or substituted). In particular, the decision to prescribe by brand cannot be defaulted by the system under the regulations

If you do not substitute or close off all alerts as shown in Edit protocol, when you go to generate a script, you will receive this alert.

The alerts will show if a new Infusion Order, Public Hospital Script and DHS Community Pharmacy Script is selected. It does not affect Drug Administration Chart printing (or adding nurse drugs to the chart) or the Treatment Protocol (which is not a script format).
The above 3 printed scripts will have the generic in front of the brand for the vast majority of branded medications.
To prevent a lot of alerts appearing at the point of prescribing, please make your active ingredient prescribing choice before leaving the New/Edit protocol page.
We recognise that the inclusion of these new rules is going to result in a lot of new alerts appearing and annoying users. Unfortunately, as the legislation is mandated, we do need to include at the minimum the “is brand clinically necessary” function (with the exemption alerts being strongly recommended).
In a future release, we’ll be looking at streamlining medication selection to reduce the number of clicks to create a valid script at which point we’ll be reviewing the Active Ingredient Prescribing function
New Menu Item - Unsigned Protocols
The new Unsigned Protocols module is linked to the logged-in User, displaying a list of all the user's patients with unsigned protocols. The menu item will appear with an ‘exclamation icon’ if the user has any unsigned protocol cycles.
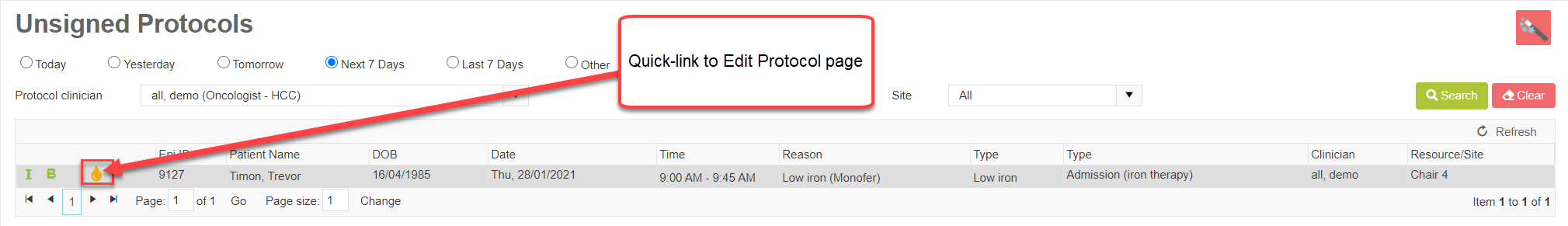
The page looks similar to the Appointment Management list, with the same search options and a teardrop icon that links to Edit Protocol so the cycle can be reviewed and signed. It also includes other useful icons, such as a direct link to the patient’s test results.
The page will have the search parameters set to ALL by default for all administrative and non-clinical staff using the module.
The list will query the protocol clinician, not the primary clinician unless they are the same person. If a locum has prescribed the protocol, the clinician who needs to sign the cycle can edit the signatory on Edit Protocol. After signing the cycle, the user will be returned to the Unsigned Protocols page with the singed cycle having dropped off the list.
Phone Orders
An upgrade to the Unsigned Phone/Standing Orders and the nurse drug chart has been implemented to enable more accurate tracking of Phone Orders. In the nurse drug chart, if a user selects phone there is now a place for the 'Heard by' nurse and the 'Confirmed by' nurse to sign. These signatures appear UNDER the phone symbol and are separate to the nurse signatures for drug administration.
For the second nurse to sign and/or if you need to edit the doctor that needs to sign, click the Edit pencil on the far right of the drug chart.
The 'Unsigned Medications' module (Unsigned Phone/Standing orders has also been upgraded to support a more user friendly search as well as display the Heard By and Confirmed by nurse signature columns for Phone orders rather than the columns that were in this module previously (of nurse administration signatures)
Bug Fixes:
Printing HTML only Test Results:
When trying to print HTML only test results, users were presented with a blank pdf document. This issue has now been resolved, and HTML only test results can now be printed as expected.
Discharge Details Form – Destination Hospital disappearing:
A unique scenario was reported where the Destination Hospital recorded on the Discharge Details visit form had disappeared after saving the form. This issue has now been resolved.
This is a legislative requirement as at 1 Feb involving major changes to the medication record and the protocol module. The purpose of the legislation is to encourage generic prescribing (rather than brand medications) where it is considered safe to do so.
The ‘where it is considered safe’ has resulted in a number of drugs falling into two exemption categories:
The List of Excluded Medicinal Items (LEMI) means the drug is exempt from including generic name on the script at all. This includes drugs with four or more active ingredients, a number of vaccines, allergens and other assorted formulations. If prescribed by brand, only brand will present on the printable script. If prescribed by generic, there will be a prompt to consider prescribing by brand instead.
The second exemption category – List of Medicines for Brand Consideration (LMBC) is where brand inclusion is recommended for clinical reasons. If prescribed by brand, both brand and generic will present on the printable script for an LMBC drug. If prescribed by generic, there will be a prompt to consider prescribing by brand.
The majority of drugs fall into neither exemption category, which means as a general rule, there will be less alerts on the whole if you record and prescribe medications by generic. If you prescribe by generic, there will be overall fewer prompts and changes to your use of either the main medication or protocol module. If you prescribe by brand, you will be prompted to confirm brand name is clinically necessary. If you decide that it is, a script for brand medication will also include generic name which will appear in front of the brand name on the printed script. This is part of the mandatory legislative requirement (that the generic is on the printed script for all drugs other than LEMIs – see above for definition) so please check your printed scripts as the vast majority of new scripts created from this date onwards will now print both generic and brand.
MIMS is providing the flags that cover the above two exemptions so as you select a drug from MIMS, the check will occur to determine whether the drug has one of these exemption flags or not. There are also of course thousands of medications already recorded and/or published in a protocol definition that are part of current medical records that could also be impacted so we have flagged these in the respective modules for you to modify or decide to proceed with current format before you get to generating the script.
How has this legislative requirement been implemented in EpiSoft?
Medication form changes
On the medication (non protocol) form, historically recorded medications will display an alert if they have been recorded in a method not compatible with the Active Ingredient Prescribing regulations. This is to assist you in making your decision regarding brand or generic before you generate a new script for those drugs. See below.
If you decide to keep the medication as is, click the alert icon and then OK to keep your generic or brand decision as is, otherwise selecting cancel will open the medication in Edit mode and you can reselect the drug by generic name (or vice versa).
If you have decided to retain brand or generic as per the patient’s current list, overwriting the AIP recommendation, a green tick will show this choice has been made for this patient and that drug and the tooltip will show who ticked this and when.
If you go to write a script without actioning these alerts, the script module will prevent you from proceeding until a decision has been made (or the drug re-selected).
When you go to record a new medication, you will get a prompt as shown below. While we recognise that this also impacts sites that are merely recording current medications (and not writing prescriptions), we have included this prompt at the time of medication recording to reduce prompts and alerts at the time of script writing.
Protocol Module changes:
When you go to record a new medication, you will get a prompt as shown below. While we recognise that this also impacts sites that are merely recording current medications (and not writing prescriptions), we have included this prompt at the time of medication recording to reduce prompts and alerts at the time of script writing.
There are a number of drugs normally prescribed by generic which are actually flagged as LMBC (List of Medicines for Brand Consideration) so this will comprise the bulk of the AIP alerts (examples rituximab, trastuzumab (not all formulations), zoledronic acid.
For this reason and because the LMBC inclusion is strongly recommended (not mandated), we have ticked all LMBC drugs by default so when you save New Protocol or “Apply Medication Selection” on Edit protocol, this default tick will be saved.
To reiterate, if the patient is already on a protocol with AIP alert listing as below and the drug has been auto-ticked, you will still need to hit Apply Medication Selection to commit that default to the patients current and future protocol cycles.
The other items which are LEMI or branded medications where generic is recommended will need to be actively ticked (or substituted). In particular, the decision to prescribe by brand cannot be defaulted by the system under the regulations
If you do not substitute or close off all alerts as shown in Edit protocol, when you go to generate a script, you will receive this alert.
The alerts will show if a new Infusion Order, Public Hospital Script and DHS Community Pharmacy Script is selected. It does not affect Drug Administration Chart printing (or adding nurse drugs to the chart) or the Treatment Protocol (which is not a script format).
The above 3 printed scripts will have the generic in front of the brand for the vast majority of branded medications.
To prevent a lot of alerts appearing at the point of prescribing, please make your active ingredient prescribing choice before leaving the New/Edit protocol page.
We recognise that the inclusion of these new rules is going to result in a lot of new alerts appearing and annoying users. Unfortunately, as the legislation is mandated, we do need to include at the minimum the “is brand clinically necessary” function (with the exemption alerts being strongly recommended).
In a future release, we’ll be looking at streamlining medication selection to reduce the number of clicks to create a valid script at which point we’ll be reviewing the Active Ingredient Prescribing function
New Menu Item - Unsigned Protocols
The new Unsigned Protocols module is linked to the logged-in User, displaying a list of all the user's patients with unsigned protocols. The menu item will appear with an ‘exclamation icon’ if the user has any unsigned protocol cycles.
The page looks similar to the Appointment Management list, with the same search options and a teardrop icon that links to Edit Protocol so the cycle can be reviewed and signed. It also includes other useful icons, such as a direct link to the patient’s test results.
The page will have the search parameters set to ALL by default for all administrative and non-clinical staff using the module.
The list will query the protocol clinician, not the primary clinician unless they are the same person. If a locum has prescribed the protocol, the clinician who needs to sign the cycle can edit the signatory on Edit Protocol. After signing the cycle, the user will be returned to the Unsigned Protocols page with the singed cycle having dropped off the list.
Phone Orders
An upgrade to the Unsigned Phone/Standing Orders and the nurse drug chart has been implemented to enable more accurate tracking of Phone Orders. In the nurse drug chart, if a user selects phone there is now a place for the 'Heard by' nurse and the 'Confirmed by' nurse to sign. These signatures appear UNDER the phone symbol and are separate to the nurse signatures for drug administration.
For the second nurse to sign and/or if you need to edit the doctor that needs to sign, click the Edit pencil on the far right of the drug chart.
The 'Unsigned Medications' module (Unsigned Phone/Standing orders has also been upgraded to support a more user friendly search as well as display the Heard By and Confirmed by nurse signature columns for Phone orders rather than the columns that were in this module previously (of nurse administration signatures)
Bug Fixes:
Printing HTML only Test Results:
When trying to print HTML only test results, users were presented with a blank pdf document. This issue has now been resolved, and HTML only test results can now be printed as expected.
Discharge Details Form – Destination Hospital disappearing:
A unique scenario was reported where the Destination Hospital recorded on the Discharge Details visit form had disappeared after saving the form. This issue has now been resolved.