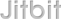Knowledge base » Release Notes - EpiSoft/CareZone » 03/11/2016 Release Notes- Clinical
03/11/2016 Release Notes- Clinical
Changes to EpiSteme Protocol Module (drug administration & prescribing)
There was an issue reported that if a nurse changed the actual dose given on the drug admin chart this was not visible in the 'dose change history' and could only be seen by viewing the drug administration chart. This issue has been resolved and changes made by nurses to the dose given will be displayed in the dose change history.
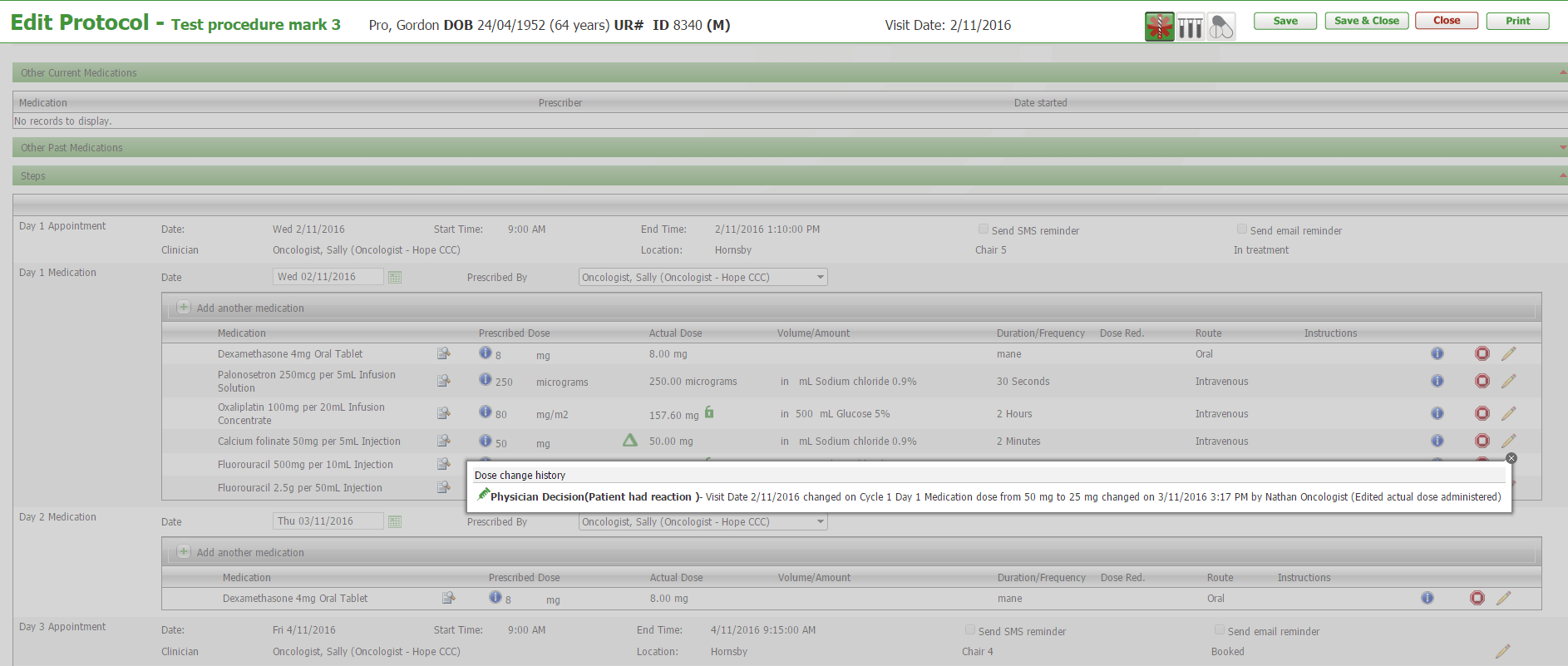
The sign & cosign check-boxes have been moved to the left side of the drug administration grid so that they are now next to the drug name which will be of benefit to users that choose to zoom their browser as they will no longer have to scroll to the right side of the page to sign medications.
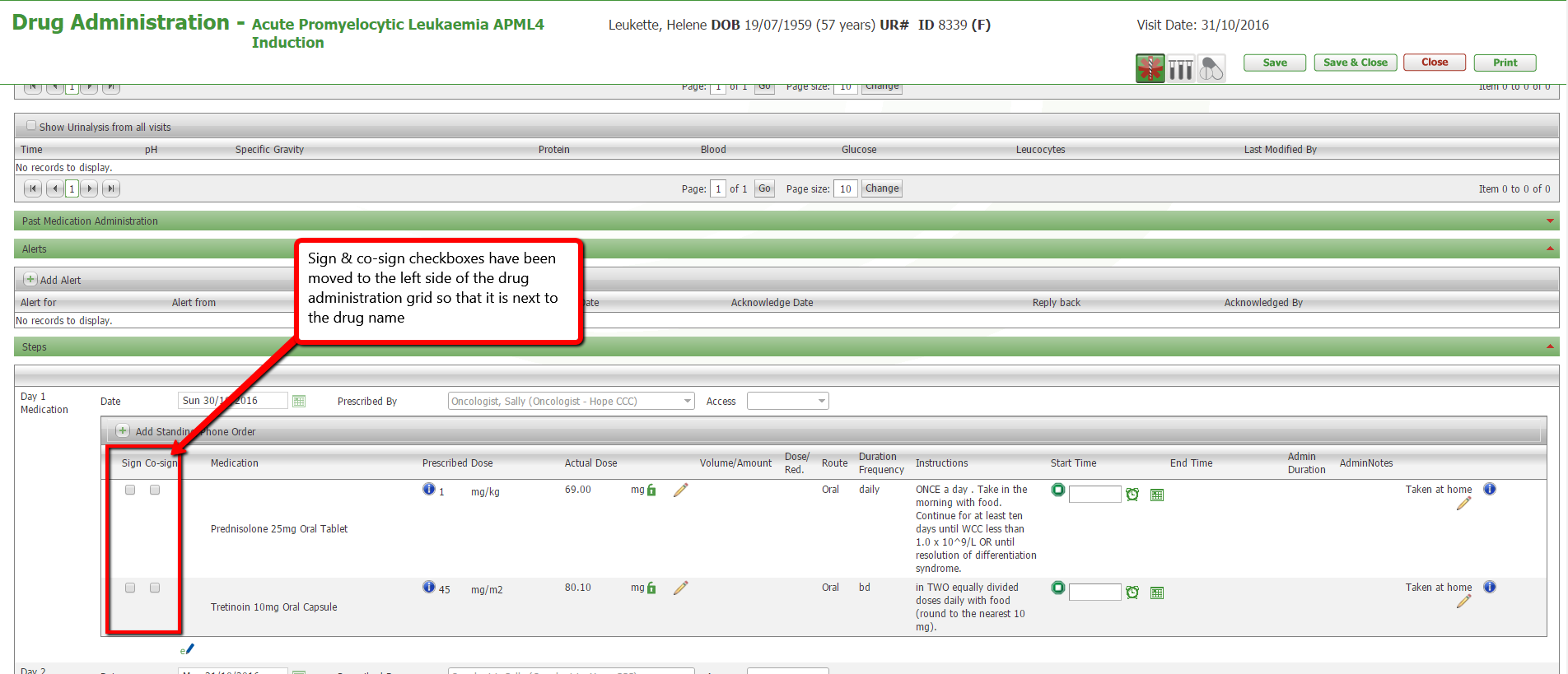
The manually entered Se Creatinine results dialogue box did not have a cancel button. This has now been resolved.
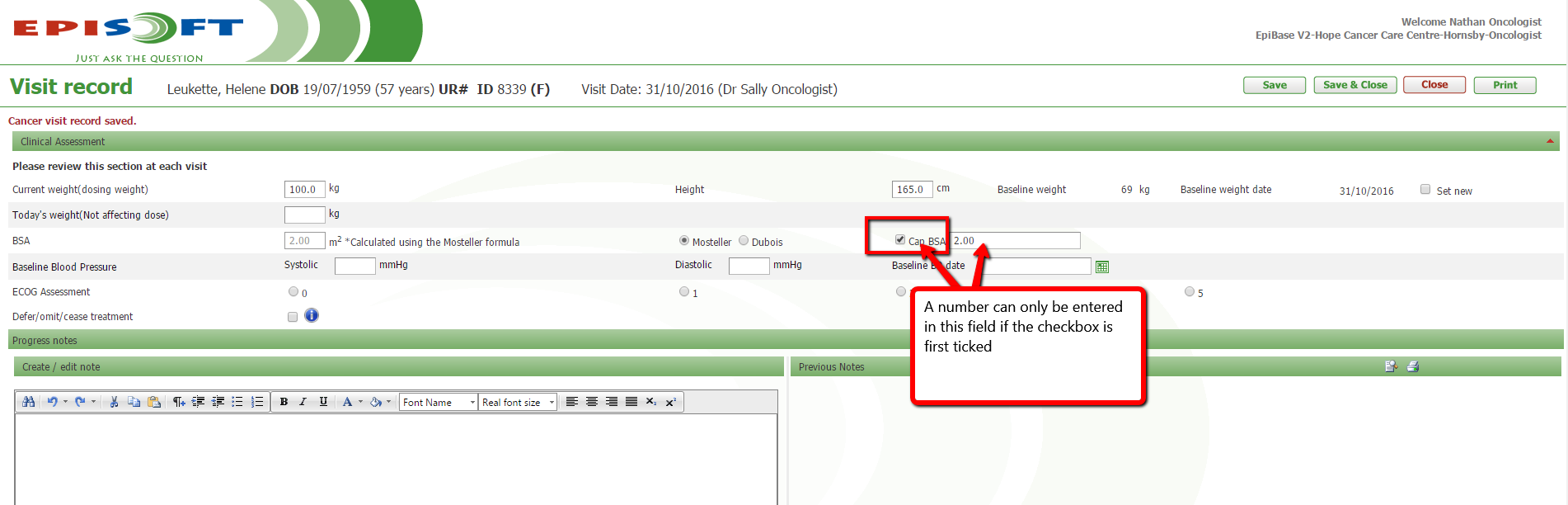
An usability issue was reported regarding the BSA capping. If a user entered the number to cap the BSA at but did not check the 'Cap BSA' box this would not cap the BSA and was confusing users. We have now put a validator in place so that users can only enter a cap BSA number if the checkbox is first ticked.
Changes to EpiSteme Protocol Authoring Module
The protocol publishing module was allowing users to publish protocols with incomplete step data- for example a procedure step that did not have a procedure type selected. We have put a validator in place to prevent this occurring in the future.
An issue was reported about 'Appointment notes' created in protocol authoring not copying forward when additional cycles of the protocol were prescribed. This issue has been resolved
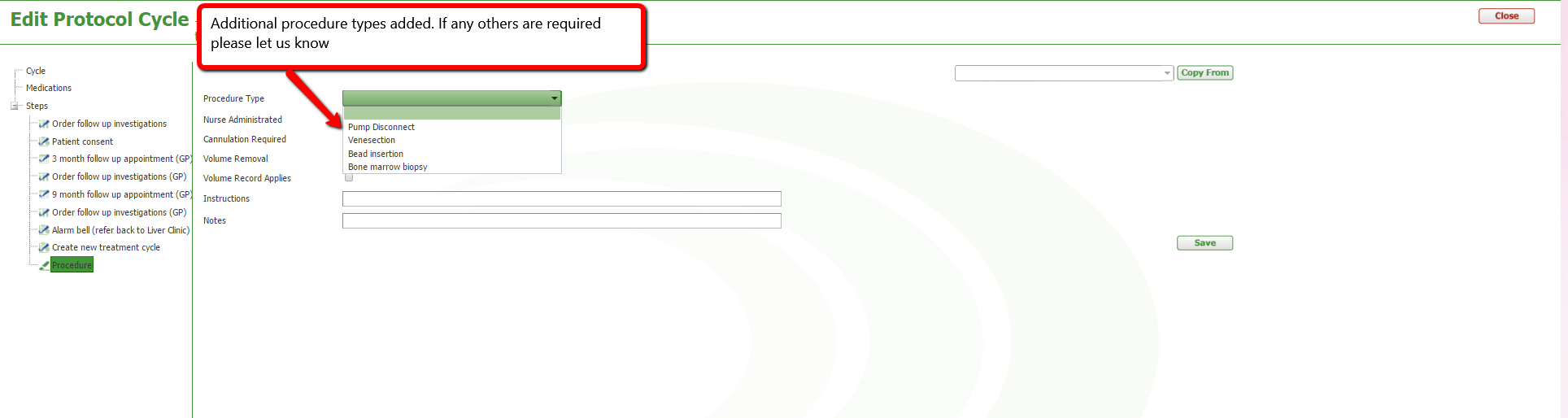
We have added 'Bone Marrow Biopsy' & pump disconnect to the list of procedure types in the protocol authoring module. If you require any additional procedure types please let us know by emailing help@episoft.com.au
Changes to EpiSteme Protocol Module- Scheduling
An issue was detected that If a user changed the date of a step in a protocol that preceded an already attended appointment and the user chose to recalculate future steps this would move the already attended appointment. This issue has been resolved, appointments/visits will no longer be moved by recalculating a preceding step.
Phone and Standing order signature within 24 hours or prescribing
Doctors are now able to sign for phone and standing orders to enable hospitals to meet their legal requirements that these orders be signed within 24 hours of being created by the nurse.
There are 4 main new functions:
· Dr eSigning via the Drug Admin chart
· Dr eSigning via a new menu item in the main menu – item drops off worklist when signed
· Email notification of new drug that requires signature sent to prescribing clinician
· This function ONLY applies to standing and phone orders and not to nurse initiated
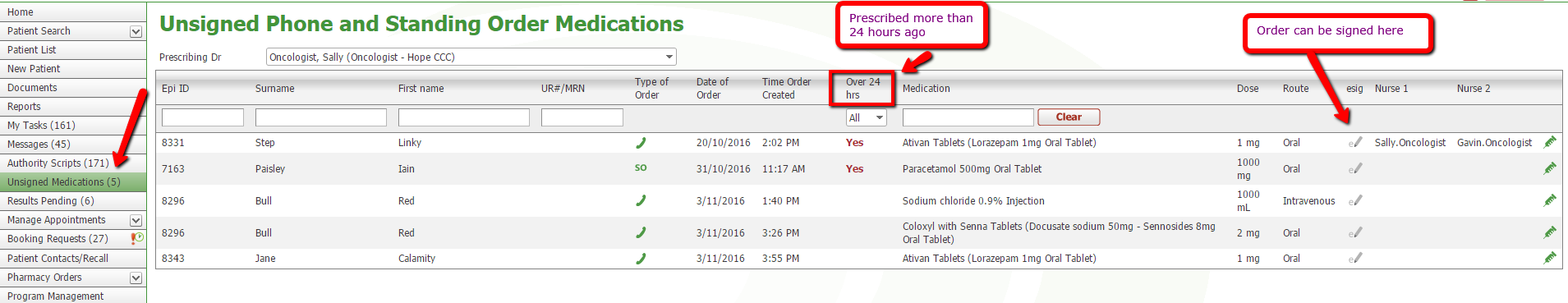
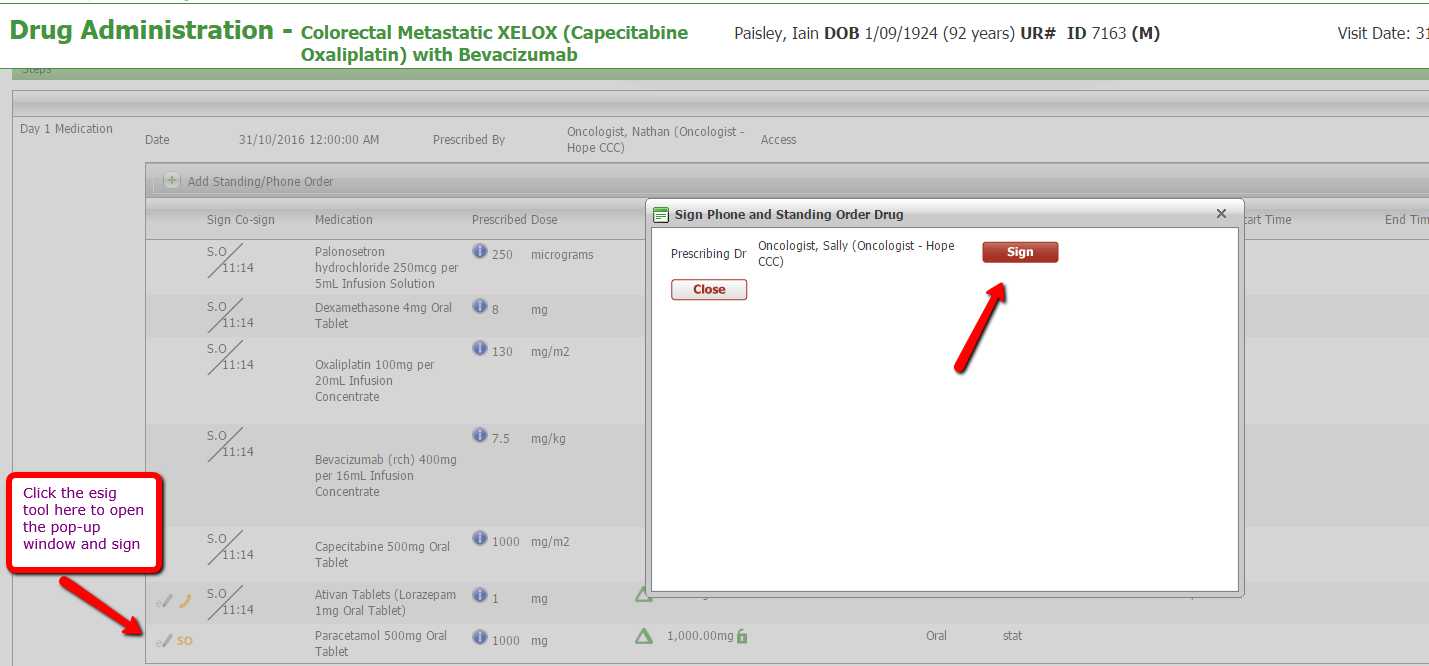
Clinicians will see the new menu item on the left. If a nurse adds a Standing or Phone order medication, an email will be sent to the prescribing clinician. On logging in, there will be a number next Unsigned Medications and on opening it will display medications requiring signing by that clinician. Clinicians can sign by clicking the esig tooltip which works similarly to signing for a protocol cycle, or the green syringe can be clicked to open Drug Administration in order to see the medication addition in context. The added medication can also be signed from Drug Administration.
Menu Item – Unsigned Phone and Standing Order Medications
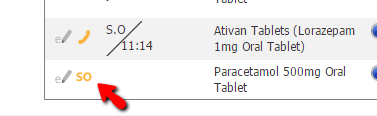
The Standing or Phone Order symbol on Drug Administration is orange when unsigned but changes to green once signed.
Signing via Drug Administration
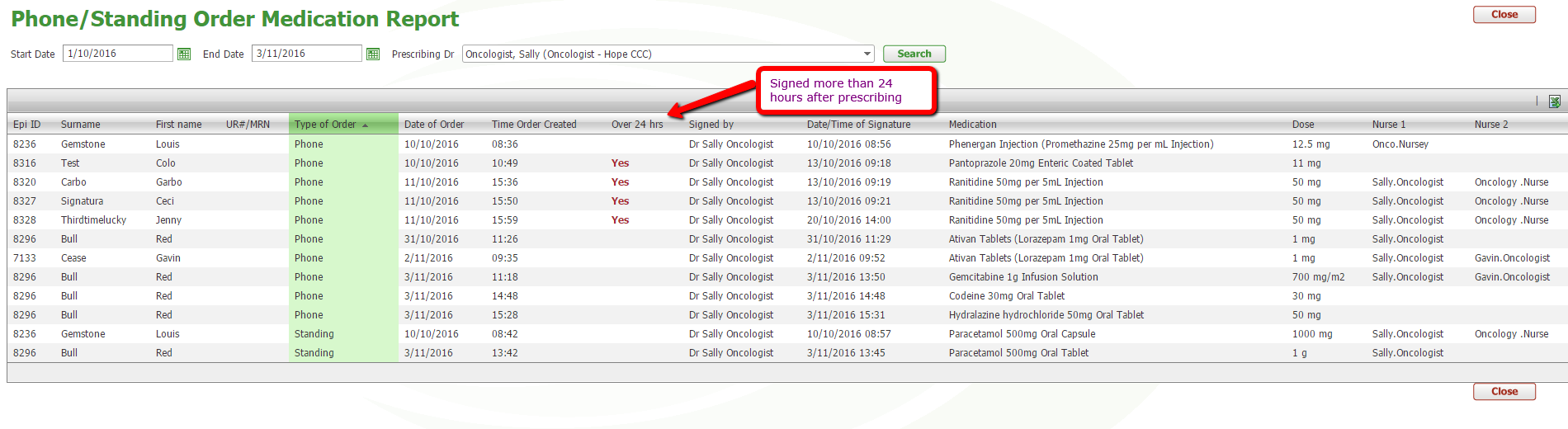
Report of Signed Medications
A report to see signed phone and standing order medications has been created and can be searched by start and end date. By clicking on the headings, the list can be filtered by each heading to see order time and signature time and if the order was signed more than 24 hours after prescribing.
Recall module
For sites using the Recall module, we now have a number of new filters on the module including:
Country (of last appointment)
Visit Type (with option to check multiple)
Exclude booked appointments checkbox
As well as a quick link to the patient private notes from the recall record.
New Clinical Reports
Treatment variance report: A new report has been created that records dose changes relative to the protocol definition (base dose). If the same change is copied, this records a first occurrence date, last occurrence date and a count of occurrences for that change. The last column records the mechanism for the dose variance e.g. change of prescribed dose, Dr locked dose, BSA capped etc. The report date range is first occurrence dates within the date range. This is designed to enable sites to review treatment variances that occurred within a defined review period.
Cytotoxic User Report: A new report has been created for sites administering chemotherapy. The 'Cytotoxic Use' report records the potential exposure to the cytotoxic drug by the nurses. It is based on the First Nurse column who signed and the dose administered. There is a field to record any details about exposure in the nurse assesment which will display against any cytotoxic drugs administered to that patient on that day. For a drug to appear in this report, the Pharmacy Admin module needs to have "Is cytotoxic" checkbox ticked. Matching routine is the same as for Pharmacy Orders and could be subject to change with updates to MIMS and removal of originally used drug identifiers.
Hepatitis Raw Data Report: A new report has been created for B In IT program users. This report is called the 'Hepatitis Raw Data'. The reports displays nearly all data that is captured on the 'Hepatitis B Assessment Form' for all records with data entered on that form.
Hepatitis history and assessment visit form now has a flag for confirmed related diagnoses of HCC and decompensated liver. These are also included in the above report.
There was an issue reported that if a nurse changed the actual dose given on the drug admin chart this was not visible in the 'dose change history' and could only be seen by viewing the drug administration chart. This issue has been resolved and changes made by nurses to the dose given will be displayed in the dose change history.
The sign & cosign check-boxes have been moved to the left side of the drug administration grid so that they are now next to the drug name which will be of benefit to users that choose to zoom their browser as they will no longer have to scroll to the right side of the page to sign medications.
The manually entered Se Creatinine results dialogue box did not have a cancel button. This has now been resolved.
An usability issue was reported regarding the BSA capping. If a user entered the number to cap the BSA at but did not check the 'Cap BSA' box this would not cap the BSA and was confusing users. We have now put a validator in place so that users can only enter a cap BSA number if the checkbox is first ticked.
Changes to EpiSteme Protocol Authoring Module
The protocol publishing module was allowing users to publish protocols with incomplete step data- for example a procedure step that did not have a procedure type selected. We have put a validator in place to prevent this occurring in the future.
An issue was reported about 'Appointment notes' created in protocol authoring not copying forward when additional cycles of the protocol were prescribed. This issue has been resolved
We have added 'Bone Marrow Biopsy' & pump disconnect to the list of procedure types in the protocol authoring module. If you require any additional procedure types please let us know by emailing help@episoft.com.au
Changes to EpiSteme Protocol Module- Scheduling
An issue was detected that If a user changed the date of a step in a protocol that preceded an already attended appointment and the user chose to recalculate future steps this would move the already attended appointment. This issue has been resolved, appointments/visits will no longer be moved by recalculating a preceding step.
Phone and Standing order signature within 24 hours or prescribing
Doctors are now able to sign for phone and standing orders to enable hospitals to meet their legal requirements that these orders be signed within 24 hours of being created by the nurse.
There are 4 main new functions:
· Dr eSigning via the Drug Admin chart
· Dr eSigning via a new menu item in the main menu – item drops off worklist when signed
· Email notification of new drug that requires signature sent to prescribing clinician
· This function ONLY applies to standing and phone orders and not to nurse initiated
Clinicians will see the new menu item on the left. If a nurse adds a Standing or Phone order medication, an email will be sent to the prescribing clinician. On logging in, there will be a number next Unsigned Medications and on opening it will display medications requiring signing by that clinician. Clinicians can sign by clicking the esig tooltip which works similarly to signing for a protocol cycle, or the green syringe can be clicked to open Drug Administration in order to see the medication addition in context. The added medication can also be signed from Drug Administration.
Menu Item – Unsigned Phone and Standing Order Medications
The Standing or Phone Order symbol on Drug Administration is orange when unsigned but changes to green once signed.
Signing via Drug Administration
Report of Signed Medications
A report to see signed phone and standing order medications has been created and can be searched by start and end date. By clicking on the headings, the list can be filtered by each heading to see order time and signature time and if the order was signed more than 24 hours after prescribing.
Recall module
For sites using the Recall module, we now have a number of new filters on the module including:
Country (of last appointment)
Visit Type (with option to check multiple)
Exclude booked appointments checkbox
As well as a quick link to the patient private notes from the recall record.
New Clinical Reports
Treatment variance report: A new report has been created that records dose changes relative to the protocol definition (base dose). If the same change is copied, this records a first occurrence date, last occurrence date and a count of occurrences for that change. The last column records the mechanism for the dose variance e.g. change of prescribed dose, Dr locked dose, BSA capped etc. The report date range is first occurrence dates within the date range. This is designed to enable sites to review treatment variances that occurred within a defined review period.
Cytotoxic User Report: A new report has been created for sites administering chemotherapy. The 'Cytotoxic Use' report records the potential exposure to the cytotoxic drug by the nurses. It is based on the First Nurse column who signed and the dose administered. There is a field to record any details about exposure in the nurse assesment which will display against any cytotoxic drugs administered to that patient on that day. For a drug to appear in this report, the Pharmacy Admin module needs to have "Is cytotoxic" checkbox ticked. Matching routine is the same as for Pharmacy Orders and could be subject to change with updates to MIMS and removal of originally used drug identifiers.
Hepatitis Raw Data Report: A new report has been created for B In IT program users. This report is called the 'Hepatitis Raw Data'. The reports displays nearly all data that is captured on the 'Hepatitis B Assessment Form' for all records with data entered on that form.
Hepatitis history and assessment visit form now has a flag for confirmed related diagnoses of HCC and decompensated liver. These are also included in the above report.